Burrows Consulting has navigated countless healthcare providers through the FQHC/FQHC Look-Alike application and site visit processes. One of the major perks of participating in the Health Center Program (HCP) is access to the 340B Drug Pricing Program as a Covered Entity. In 2022, our firm began working with several healthcare providers seeking alternative eligibility and participation in the 340B Program through specializing in STD, HIV and Hepatitis care for vulnerable and at-risk populations in their community. Since then, Burrows Consulting has played an integral role in establishing STD clinics in multiple states for varying entity types, including organizations with a for-profit tax classification.
What is the 340B Drug Pricing Program?
The 340B Program turned 30 years old last year. Created in 1992 to ensure that at-risk, underserved, uninsured and other vulnerable populations have access to critical drugs. Decreasing cost barriers for eligible drugs have allowed providers to deliver better clinical outcomes.
The 340B world has seen a large amount of controversy in recent years, ranging from manufacturers attempting to dismantle the Program through lobbying efforts and refusal to ship to contract pharmacies, third-party administrators heavily taxing the industry with software and providers exposed for poor allocation of revenue achieved through 340B drug cost savings. Remember, the primary objective of the Program is to spread scarce federal dollars to those with challenges accessing it.
What is an STD Clinic?
So called ‘STD Clinics’ officially broke ground in 1979, but treatment and screening protocols and industry norms have changed tremendously in 43 years. States (and non-governmental organizations) receive CDC grants aimed at STD control & elimination under Section 318 of the Public Health Service Act.
We will share some of the common pains that we’ve heard from providers in their attempts to diagnose and treat STDs, HIV, hepatitis and in their community:
-
Price fluctuations on key screening and treatment supplies (including medically necessary prescription medications)
-
Supply shortages, including long-term global supply chain issues that spiraled out of control due to the global pandemic in 2020
-
The excessive cost of providing additional services required to diagnose and manage comorbidities correlated or caused by HIV, STDs, TB and HEP. Examples include:
o Behavioral Health Services
o Chronic Care Management
o Intensive Outpatient Services
o Substance Use Disorder Services
Participation in the 340B Program allows providers an additional stream of revenue through cost savings generated when patients are dispensed 340B-eligible drugs at organization-owned, in-house and contract pharmacies. Providers invest these savings back into the patient through providing expanded and enhanced services.
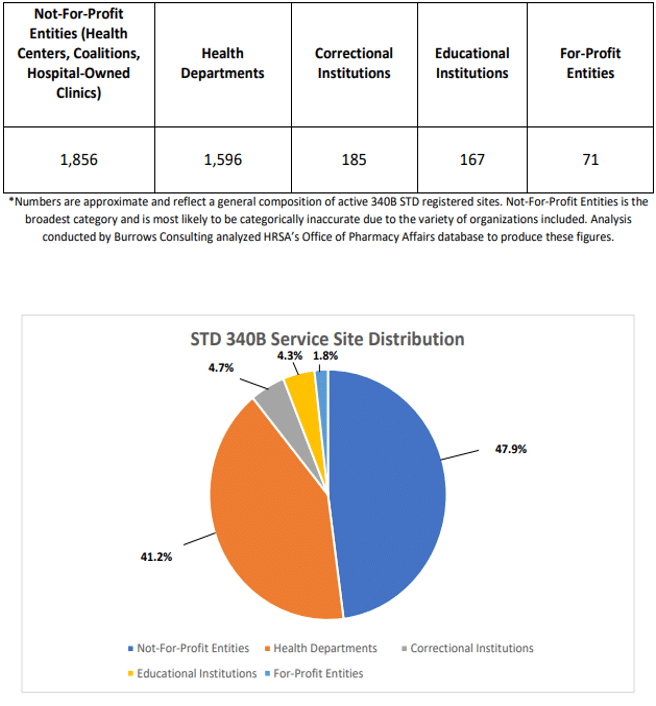
Data Source: Office of Pharmacy Affairs, 2022; interpretation of data by Burrows Consulting
Eligibility
While our firm works with a variety of organizations seeking different 340B designations, we will examine STD Clinic eligibility for the purposes of this publication. HRSA’s Sexually Transmitted Disease Clinics webpage is not very helpful when it comes to specifics about eligibility, but we have learned a few things about what is needed to establish a HRSA-approved Sexually Transmitted Disease Clinic:
-
Health Departments, Community Health Centers and other private not-for-profit organizations make up most of the STD participants, but we have identified more than 70 for-profit entities registered for the 340B Program as STD clinics in Pennsylvania, Ohio, Florida, Indiana, Nebraska, Kentucky, Louisiana, Oklahoma, Arizona, Nevada and Colorado. Some of these entities also participate in the Program as a Ryan White grantee.
-
Eligibility for private entities requires either a direct grant from the CDC or an agreement with the entities’ state, such as a Memorandum of Understanding outlining the provider’s scope of services and deliverables relating to STD screening and treatment.
-
This distinction is known as “Direct Funding” vs. “In-Kind Support”, a criterion that HRSA uses to verify federal grant connection when registering the entity in HRSA’s Office of Pharmacy Affairs database.
-
HRSA has left most eligibility standards and participation requirements to the states, but HRSA is the final decision maker after a state has approved the candidate.
-
Established organizations (or new organizations that branch off of an established organization) that serve at-risk populations, large Medicaid populations, uninsured and underinsured are preferred.
-
The state or community has a need for expanded services due to population increases, increased prevalence of infectious diseases and other risk factors.
-
Many states have community partnerships in place already, but some, unfortunately, do not.
Burrows Consulting helps entities become eligible for STD or HIV designations through direct grants and in-kind support. Our firm offers free initial consultations regardless of your state and entity type (Governmental, For-Profit, Not-For-Profit, Rural Health Clinic, FQHC, FQHCLA, CMHC, CCBHC, etc). If your organization is interested in learning more about how to establish an STD, Hepatitis or HIV clinic, please fill out our contact form and a member of our team will be in touch.